A quick scene-setter (coffee optional)
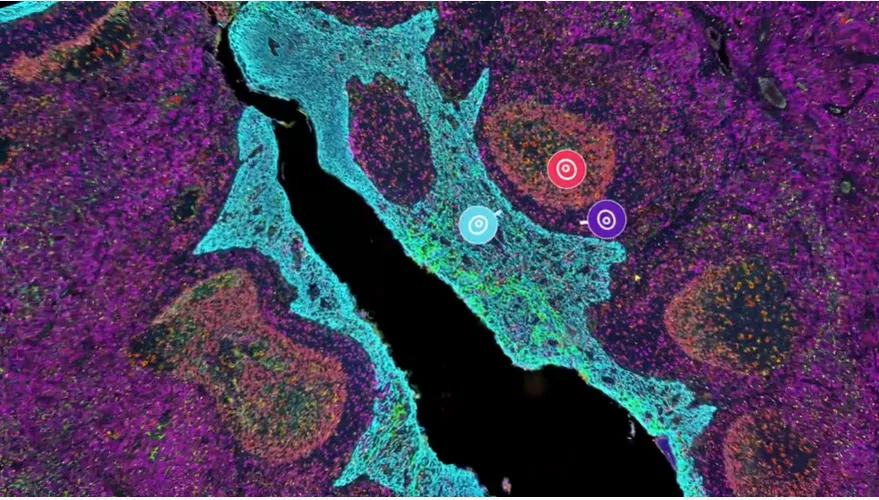
Cancer biologists like to call the tumor a “mass.” In reality it behaves more like Times Square on New Year’s Eve: a jostling crowd of tourists (immune cells), cops (fibroblasts), food trucks (blood vessels), pickpockets (cancer-associated macrophages) and the giant LED billboards of cytokines blaring contradictory instructions. That carnival is the tumor microenvironment (TME)—the ecosystem within ~200 µm of every malignant cell. The term itself dates back to Judah Folkman’s angiogenesis work in the late 1970s, but it gained real momentum only after stromal biology exploded in the early 2000s. Today PubMed logs >58 000 hits for “tumor microenvironment,” with citations doubling roughly every four years. WikipediaMD Anderson Cancer Center
What exactly is the TME?
At its simplest, the TME = cancer cells + everything they touch. That includes:
| Neighborhood resident | What they bring to the block | Typical lab marker |
|---|---|---|
| Immune cells (T & B lymphocytes, NK, macrophages) | Police force, occasionally on the take | CD3, CD8, CD68 |
| Cancer-associated fibroblasts (CAFs) | Remodel the extracellular matrix (ECM), secrete growth factors | α-SMA, FAP |
| Endothelial cells & pericytes | Build leaky blood/lymph vessels | CD31, PDGFR-β |
| Extracellular matrix | Collagen, hyaluronan, stiffness cues | Collagen I/III |
| Metabolic gradients | Hypoxia, acidity, lactate | HIF-1α, CAIX |
| Nerves & adipocytes | Niche signalling, fatty-acid fuel | β-III tubulin, PLIN1 |
These players talk through cytokines, exosomes and direct cell–cell contacts, continuously re-writing the local rulebook. The net effect is to promote tumor survival, invasion and—cruel irony—therapy resistance. Wikipedia
Cold, Hot, Excluded, Desert: the Weather Forecast for Your Tumor
Immuno-oncologists can’t resist meteorology metaphors, and for good reason: they capture, at a glance, how friendly—or hostile—a tumor’s neighborhood is to infiltrating T cells. Here’s the fuller forecast:
Hot tumors
- Definition: Robust CD8⁺ and CD4⁺ T-cell infiltrates are embedded deep inside malignant nests, often co-localized with activated dendritic cells (“immune inflamed” phenotype).
- Clinical vibe: These lesions already look like small immunological brushfires, so a little immune-checkpoint gasoline (PD-1, PD-L1, CTLA-4 blockade) can ignite a therapeutic inferno. Melanoma and NSCLC responders typically live here.
- Molecular hallmarks: High interferon-γ signature, elevated CXCL9/10 chemokines, up-regulated MHC-I/II, and plenty of clonal neo-antigen TCRs.
- Emerging twists: Even “hot” tumors can run out of fuel—chronic antigen exposure exhausts T cells. Combo trials now layer PD-1 blockade with IL-2/IL-15 agonists, TIGIT or LAG-3 inhibitors to re-energize the firefight.
Cold / Immune-desert tumors
- Definition: Cellular tundra—lymphocytes are scarce or absent throughout the parenchyma and stroma.
- Why so barren? Poor antigenicity (few mutations), defective antigen presentation, “don’t come here” chemokine profiles, or physical barriers like dense collagen.
- Conversion strategies:
- Oncolytic viruses—infect, lyse, and shout danger signals (e.g., T-VEC + pembrolizumab trials).
- STING or TLR agonists—synthetic alarms that recruit dendritic cells.
- Epigenetic drugs—demethylate tumor DNA to reveal cryptic antigens.
- Radiation “in situ vaccine”—creates local debris and type-I interferon splash.
- Benchmark: A cold melanoma can jump two immune quartiles after a single fraction of stereotactic radiation plus a STING agonist, according to recent phase-I readouts.
Immune-excluded tumors
- Definition: T cells march up to the castle wall (stroma) but never breach the inner keep. Think of them as fans tailgating outside the stadium.
- Key barricades:
- TGF-β-driven fibroblast webs that crosslink collagen into a Kevlar-grade matrix.
- VEGF-induced abnormal vasculature—vessels are leaky yet paradoxically poor at allowing lymphocyte trafficking.
- Chemokine mismatch—CXCL12 pulls T cells to the perimeter, CXCL9/10 needed inside are missing.
- Breaking the siege:
- Bintrafusp alfa (PD-L1 × TGF-β trap) and anti-TGF-β antibodies aim to dissolve the ECM moat.
- Low-dose VEGF inhibitors (bevacizumab, lenvatinib) normalize vessels, opening new gates for T cells.
- CAR-T or TCR-T cells engineered with heparanase or CXCR2 are in early trials, designed to chew through stroma or follow tumor-secreted chemokines.
Hybrid & transitional states
Tumors aren’t static climates; a “hot-but-exhausted” or “altered-immunosuppressed” state often sits between categories. Serial biopsies and spatial transcriptomics now show micro-islands of heat surrounded by cold estuaries—revealing why a 1 cm core needle can mislead treatment choices.
Why the re-weatherization obsession?
Turning cold or excluded lesions into bona fide hotspots has become a marquee R&D objective. By 2025, >150 active trials combined checkpoint inhibitors with TME-modulating agents—oncolytic viruses, VEGF/TGF-β blockers, STING or CD40 agonists—each aiming to rewrite the local forecast. The ultimate goal: make “sunny with scattered T cells” the default outlook for every patient.
Why drug developers obsess over the TME
- Resistance insurance
Checkpoint blocker not working? Look for CAF-built collagen barricades physically keeping T cells out or myeloid-derived suppressor cells soaking up every spare interferon. - Metastasis control room
The “pre-metastatic niche” theory—where primary tumors remote-control bone-marrow-derived cells to prep distant soil—has moved from wild idea to textbook fact. - Biomarker treasure chest
Spatial patterns (e.g., a 10 µm “death zone” of exhausted CD8s at the tumor–stroma border) predict response better than any single gene. Venture capital has noticed. - New drug targets
TME-centric assets now make up roughly 35 % of early-phase oncology trials (think VEGF, CSF-1R, LAG-3, TGF-β, adenosine). The FDA cleared nine TME-modulating agents in Q4 2024 alone. AACR
A (very) short history of spatial transcriptomics
2016, Stockholm. Patrik Ståhl, Joakim Lundeberg and colleagues glue barcoded oligo spots onto a slide, overlay a tissue section, then sequence the cDNA. Voilà: spatial transcriptomics (ST)—gene-expression heatmaps that know exactly where they came from. Science
Within three years 10x Genomics licensed the idea as Visium, quickly followed by:
- MERFISH from Harvard/Vizgen (1 000 + genes imaged by sequential FISH)
- CosMx SMI from NanoString (fully automated, FFPE-friendly)
- DBiT-seq from Stanford (microfluidic nanobars across the slide)
And universities? Take your pick: Karolinska, Broad, MIT, Stanford, Johns Hopkins—each has dedicated spatial cores churning out datasets the size of Netflix’s billing logs. 10x GenomicsPubMed Central
Why “spatial” is the TME’s best paparazzi
Conventional single-cell RNA-seq tears cells out of context—great for doing a census, terrible for understanding neighborhood zoning laws. ST stitches the two together, letting scientists:
- Map immune ecotypes (inflamed vs. excluded vs. desert) in situ.
- Trace metabolic gradients—e.g., lactate pools that turn macrophages M2-like within 50 µm.
- Watch therapy-induced rewiring—TRACERx studies show how checkpoint blockade reshuffles cell–cell talk. PubMed Central
A 2024 pan-cancer meta-analysis combined 1.2 million spatial spots with ATAC-seq to reveal six conserved stromal programs—two of which (ECM-remodelling CAF-B and angiogenic CAF-E) correlate with poor immunotherapy outcome across 11 tumor types. Nature
The TME as a drug target: case studies
| Pathway | Rationale | Agent(s) in clinic | Fun fact |
|---|---|---|---|
| TGF-β | Fibrosis, immune exclusion | M7824 (bintrafusp alfa), galunisertib | Dual PD-L1/TGF-β trap had mixed results—still alive in HPV cancers |
| CSF-1R | Depletes M2 TAMs | Pexidartinib (FDA-approved for TGCT), emactuzumab | Pexidartinib is first-in-class TME drug not aimed at cancer cells at all |
| HIF-2α | Hypoxia survival | Belzutifan (ccRCC) | Approved 2021; now tested as radiosensitiser |
| CXCR4 | Immune-cell trafficking barrier | Motixafortide, mavorixafor | Often combined with anti-PD-1 in “barrier-buster” trials |
| LOX / collagen crosslinking | ECM stiffening | Simtuzumab, PAT-1251 | Stiff stroma = high interstitial pressure; loosening it improves drug perfusion |
Spatial buzzwords biotech investors now slip into meetings
- “Neighborhoods” – clusters of cell types that co-occur in <50 µm patches.
- “Ecotopes” – recurrent spatial ecosystems, e.g., ‘T cell & IL-17 CAF’ Island.
- “Ligand–receptor connectome” – network of inferred cell talk; hot in AI circles.
- “Spatially resolved multi-omics” – transcript + protein + chromatin + metabolites on same slice (yes, people are trying).
- “Digital pathology 2.0” – image-plus-RNA co-registered for FDA-grade biomarkers.
Expect those phrases to pop up in the next 10-K you read.
University labs pushing the envelope
| Institution | Cool project | Take-home |
|---|---|---|
| Stanford (Bertozzi & Qi labs) | CRISPR perturbations tracked by xyz-encoded barcodes in xenografts | Know not just what gene drives immune evasion, but where it matters |
| Karolinska/SciLifeLab | 3-D ST on tissue cylinders | First draft of a volumetric TME atlas |
| Johns Hopkins | Spatial proteomics on pancreatic cancer via DSP | Identifies fibroblast “girdles” that resist FAK inhibitors |
| MIT Koch Institute | Integrates ST with optical metabolic imaging | Maps hypoxia niches in real time, no dyes required |
These academic proofs-of-concept often spin out—note Dealroom’s 2025 list of 47 startups with “spatial” in their pitch decks, raising a combined $3.8 billion.
Humor break: the TME’s unwritten sitcom rules
- Everyone hates the new neighbor – NK cells move in, CAFs throw collagen at them.
- HOA fees are sky-high – angiogenic vessels charge 20 % oxygen tax, still leak.
- Recycling day is chaos – macrophages flip M1/M2 labels more often than an Airbnb.
(Back to seriousness.)
Spatial tech shopping list for biopharma R&D directors
| Platform | Max genes | Resolution | Throughput | Sample type | Best for |
|---|---|---|---|---|---|
| 10x Visium | Whole transcriptome | 55 µm spots | 1–2 slides/day | FFPE & fresh | Broad profiling |
| CosMx SMI | 6 000 proteins + RNAs | Single-cell (1 µm) | 25 mm²/day | FFPE | Target validation |
| Vizgen MERFISH | 1 000–10 000 RNAs | 0.2 µm | 500 cells/min | Fresh frozen | Cell–cell gradients |
| NanoString GeoMx DSP | 18 000 RNAs + 100 proteins | 10–600 µm ROIs | 10 slides/day | FFPE | Clinical biomarker pilots |
Prices range from $250 k hardware (Visium) to $750 k (GeoMx + NGS workflows). Reagent costs run $300–600 per cm²—cheap compared with a failed Phase III.
Regulatory & commercial outlook
- The FDA’s 2025 draft guidance on digital pathology companion diagnostics explicitly names “spatial gene-expression signatures” as acceptable evidence for enrichment trials.
- CMS’s Transitional Coverage for Emerging Technologies pathway now reimburses ST-based assays used to select immunotherapy in lung cancer.
- Big Pharma is following suit: BMS, Roche and AstraZeneca each announced spatial-omics discovery deals >$80 million upfront in 2024.
Bottom line: early adopters score first-mover biomarker IP; laggards risk writing “lack of spatial context” in their next CRL.
What’s still hard (and who’s tackling it)
| Challenge | Current workaround | Who’s poking at it |
|---|---|---|
| Throughput vs. resolution | Tile multiple Visium slides; AI up-scales | Broad Institute “marine AI” models |
| 3-D reconstruction | Cryo-section serial ST | Wyss Institute “tissue cartography” |
| Temporal dynamics | Repeat biopsies (ouch) | Stanford mini-endoscopic ST chips |
| Integrating proteomics | DSP or CODEX after RNA pass | Karolinska & Abcam conjugate panels |
| Big-data analytics | Cloud GPUs; Graph Neural Nets | NVIDIA & AWS HealthOmics alliances |
Future-casting: 2025-2030
Short term (1-2 yrs) – Clinical trials begin stratifying by spatial immune ecotype (think “PD-1 blockade + CAF inhibitor if CAF-E > 25 % in margin”).
Mid term (3-5 yrs) – Point-of-care microfluidic chips deliver intra-op spatial readouts—surgeons decide margin width on the fly.
Long term (5 + yrs) – In vivo nanoparticle barcodes label secreted cytokines, enabling live TME imaging; regulatory frameworks adapt to “digital twins” of tumors guiding dose and schedule.
Final word (for now)
The TME used to be the backdrop; today it’s the screenplay, director and box-office accountant rolled into one. Ignore it and your therapy risks becoming a one-season wonder. Master it—through spatial transcriptomics, multiplex imaging, judicious target selection—and you join the growing cast of companies turning once-untouchable cancers into manageable, perhaps even curable, diseases.
Whether you’re a grad student wrestling with CAF subtypes, a venture partner chasing the next Visium-style breakout, or a clinician deciding if that hypoxia-modifier-plus-IO trial is worth a slot for your patient, remember: the map is finally catching up to the territory. And the territory is anything but flat.